As outside temperatures drop to below 0oC in the winter months, there is a risk that water recirculating in chilled systems will freeze. To prevent the water from freezing, it is often necessary to add a glycol antifreeze.
This blog post explores the different types of glycol antifreeze and how they can protect chilled water systems.
How glycol provides freeze protection
For a chilled water system to function properly, the water it contains must continuously flow. However, the pipes can often be exposed to low enough temperatures for the water to freeze. If the frozen water is allowed to expand past the size of the pipe diameter, it can impact the system's efficiency, leading to blockages, leaks, and even burst pipes. By adding glycol to the system, you can reduce the water's freezing point and keep the water flowing, even in the coldest months of the year.
The different types of glycol
Two main types of glycol antifreeze are used as standard within the water treatment industry:
Monoethylene glycol (MEG) - This is the more cost-effective solution, but is highly toxic. About 125ml MEG is the lethal dose for a typical-sized adult. For this reason, mono propylene glycol (USP grade) should be chosen for any application where there is a possibility of contact with food, pharmaceuticals or humans.
Monopropylene glycol (MPG) – This is a pharmaceutical-grade glycol with a specified purity of greater than 99.8%.
It is important to note that glycols, particularly their breakdown products, are corrosive to most metals, so they should always be used with an appropriate corrosion inhibitor.
How the glycol level impacts chilled water systems
The level of either MEG or MPG within a system will affect a water system in several ways:
- The freezing point of the solution.
- The biostability of the system.
- The thermal conductivity/heat transfer capacity of a system.
- The viscosity of the solution to be pumped around the system.
Here, we take a look at each of these factors in turn.
1. The freezing point of the solution
Most standard specifications that include MEG will state a concentration of MEG at 25% w/w concentration within the system. This will bring the freezing point down to -12oC, which is suitable for most UK-based situations.
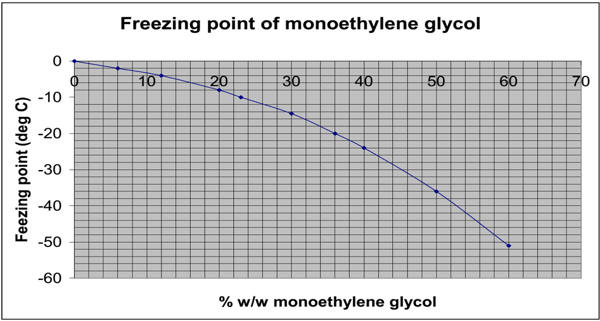
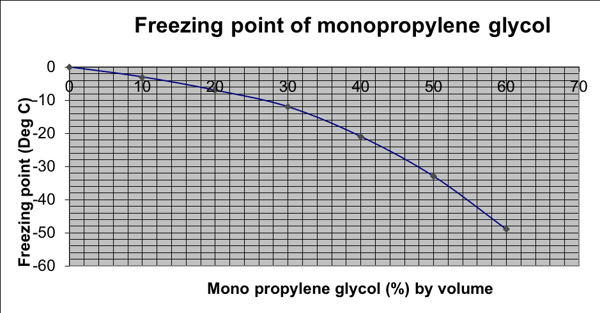
The freezing point depression of MPG is slightly less than that achieved by the addition of MEG. A 30% w/w solution of MPG is required to depress the freezing point of the system down to -12oC.
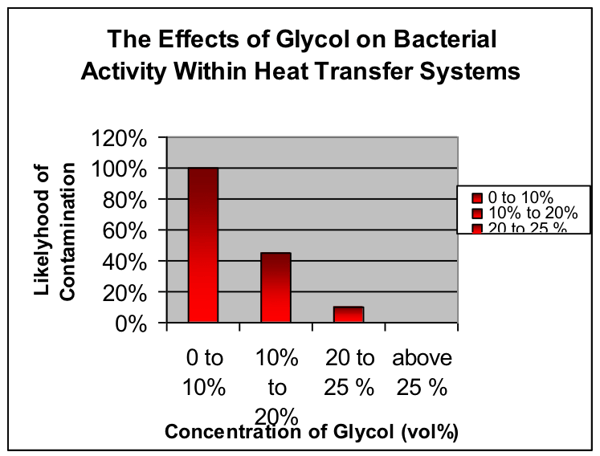
2. The biostability of the system
When closed circuits contain a minimum of 20% (but preferably 25% MEG or MPG) the glycol has a biostatic effect, preventing the growth of bacteria. As concentration falls substantially below 25%, glycol can act as a food source for bacteria. As bacteria consume the glycol, they produce acidic bi-products, namely glycolic, glyoxylic, formic, carbonic and oxalic acids, thus lowering the pH of the system. This can, in itself, lead to corrosion.
3. The thermal conductivity/heat transfer capacity of a system
Glycols can be used to decrease the thermal conductivity of the resultant solution. 20% MEG will decrease the thermal conductivity by approximately 10%, while 20% MPG will decrease the thermal conductivity by approximately 17%.
4. The viscosity of the solution to be pumped around the system
Glycols can also be used to increase the viscosity of the resultant solution. This can affect pump horsepower and fluid flow and may increase power consumption as a result. 20% MEG will increase the viscosity by 50%, while 20% MPG will increase it by approximately 115%.
How to evaluate the required glycol levels
To properly evaluate the required glycol levels within a system, it is important to understand the above impacts that the glycol levels are likely to have on the system. It is generally the case that a trade-off has to be made between system performance, biostability and freeze protection, as previously outlined.
When all the above parameters are considered, most specifications will generally include the requirement for a 25% w/w addition of glycol to a system. To ensure biodegradation of glycol does not occur, glycol concentrations should not be allowed to fall below 20% in closed-circuit systems.
How to maintain required glycol levels
It is essential to install and maintain the correct glycol concentration within a system. Too little glycol can lead to excessive microbial growth, biofilm formation and corrosion. If too much glycol is present, it can result in poor heat transfer, increased viscosity and increased energy bills, often together with damage to pumps and valves within the system.
The importance of a corrosion inhibitor - and how to choose one
The correct inhibitor must be added to the system with the glycol to prevent corrosion. Inhibited MEG and inhibited MPG are available. In these formulations, the glycol and corrosion inhibitor are combined so that at a 25% dose rate, the correct inhibitor level is also present in the system.
If MPG (USP grade) is required for a specific application, only certain approved inhibitors can be used. The same is the case for biocides.
Choosing a glycol and inhibitor
IWe have outlined the different types of glycol antifreeze products and the corrosion inhibitors they should be paired with in the table below.
| Product | Component | Purpose |
| MEG | Monoethylene glycol | General purpose antifreeze |
| MPG | Monoproptlene glycol | Food grade antifreeze |
| Inhibited MEG | MEG + molybdate-based inhibitor |
General purpose inhibited antifreeze First choice inhibited antifreeze (except for use in food factories) |
| Inhibited MPG | MPG + inhibitor |
Food grade inhibited antifreeze First choice inhibited antifreeze for food factories |
It is vital not to mix different types or brands of glycol in your chilled water system as this can cause it to congeal, clog up, and leave freeze temperatures virtually unknown.
Summary
Adding a glycol antifreeze to your chilled water system will ensure it runs efficiently. This is particularly important in the winter months when temperatures in the UK can drop to below 0oC.
MEG is a general purpose antifreeze, while MPG is specifically for use in food factories. It is important to add the appropriate biocide to prevent corrosion.
If you are considering how you could use MEG or MPG to improve the function of your chilled system and would like some advice, please feel free to contact our technical department who will be happy to help.






