Half-life (and decay rate) is an important characteristic in the dynamics of boilers and cooling systems. It is vital to be aware of it so that you can increase the dose of your biocide accordingly.
Missing the half-life out of your planning can have a serious detrimental effect on your water treatment, especially when it comes to complying with regulations such as those for Legionella control.
In this blog post, we look at the definition of half-life and explore how to calculate biocide dosing requirements so you can protect your water system and remain compliant.
What is half-life and decay rate in boilers and cooling systems?
Half-life and decay rate is the time taken for a component to reduce to 50% of its initial or starting concentration through the process of wastage – assuming no more material is being added. You may also see this written as “Holding Time Index” or HTI.
How slug of material is lost from a system
The graph below shows the way a slug of material is lost from a system, with continuous bleed and make up. It is not a straight line decrease. It is diluted more quickly at the beginning, but the time to get to ½ the strength and then ¼ and then 1/8 is the same interval (0.7h in this example).
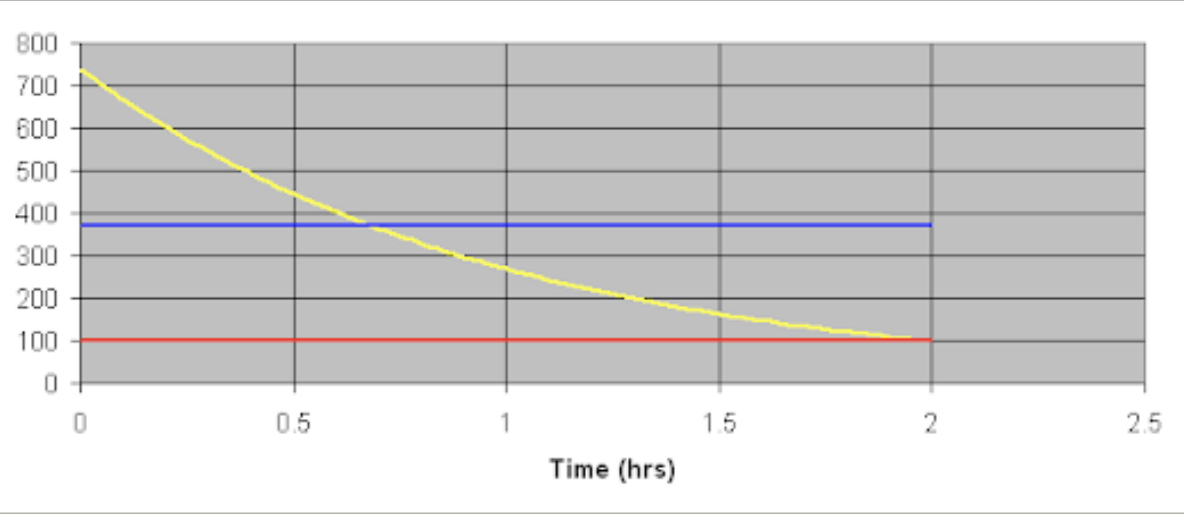
This is a mathematical function and is described as exponential decay. It is designated as “e” in mathematical formulae and does have a numerical value.
Happily, we do not need to know much more about it – except that there is a button for it on an engineering calculator! This may be labelled “e”, “ex” or “exp”.
How to calculate biocide dosing requirements
Slug dosing (or shock dosing) is the process of adding a much higher amount of chemical biocide than is normally applied in one go. The aim is to rapidly raise the concentration of biocide in the water to kill most of the organisms in the water.
When slug dosing a biocide, you can use a formula with “e” in it to calculate how much biocide you need to add to keep the dose at or above the lethal level.
The half-life is given by:
=
Where:
V= Volume of system m³
P= Purge m³/h
CF= Concentration Factor
E= Evaporation m³/h
This formula is critical to use when calculating biocide dosing requirements and can be used for understanding any slug dose situation. For example when adding antifoam to a boiler, or when purging out poor water from an upset on a boiler or a cooling system.
You can see that half-life is linked to volume and purge. The smaller the volume and the bigger the purge, the shorter the half-life and the less time chemicals are in the system. The shorter the half-life, the more chemicals needed, and the more difficult it is to control the system.
This is not only true for our chemicals but make up water parameters as well. For example, a small volume high heat load (high evaporation) system with a purge commensurate with a CF of three could have a half-life as short as four hours. If a dosing pump failed five minutes after you checked it during your service, by the next day there would be no inhibitor left in the system.
Understanding the half-life of your boiler or cooling system is an important part of your water treatment regime. It will help you protect your system and keep you compliant.
For further technical advice on half-life or biocide slug dosing please contact us on Ownlabel @bvwater.co.uk




